ARC CCeMMP Research Symposium 2025
Registration has closedDate13 Nov - 14 Nov 2025
Time 9:00 AM - 6:30 PM on 13 Nov, 9:00 AM - 4:00 PM on 14 Nov AEDT
Location Bio21 Molecular Science & Biotechnology Institute 30 Flemington Rd, Parkville, VIC 3052, Australia
The Australian Research Council funded Centre, Centre for Cryo-electron Microscopy of Membrane Proteins (CCeMMP), is hosting a free in-person 2025 Research Symposium to showcase the exciting research and technological advances in the field of cryo-electron microscopy and membrane proteins and will feature two keynote presentations. CCeMMP invite the scientific community to attend and participate in the symposium on the 13th and 14th of November in Melbourne, Australia.
This year, CCeMMP are excited to welcome two keynote speakers, presenting their recent cutting edge research, Professor Wai-Hong Tham and Associate Professor Alastair Stewart.
Prof. Wai-Hong Tham
Professor Wai-Hong Tham received her PhD from Princeton University and is an Elizabeth Blackburn NHMRC Investigator L2. She currently holds a joint appointment at the Infection and Global Health Division at the Walter and Eliza Hall Institute and at the Research School of Biology at ANU. Her lab has made fundamental discoveries in novel host-pathogen interactions and examined their molecular and structural mechanisms to drive rational design of new therapies against malaria. The overarching aim is to rationally design and generate new inhibitors or antibodies that block these interactions and stop recurrent malaria infection in humans and block transmission from mosquitoes. Her work intersects with the fields of structural biology, nanobody technology, immuno-epidemiology and molecular parasitology. For her contribution to understanding malaria parasite biology, she has received the 2023 Bancroft-Mackerras Medal, 2023 Victorian Honour Roll of Women, 2020 International Award Biochemistry Society and 2019 and 2011 Eureka Prize for Infectious Diseases Research (team prize).
Blocking Malaria Parasite Transmission: Insights from Cryo-EM of an Endogenous Fertilization Complex.
Fertilization is a critical process in generating new offspring. For malaria parasites, fertilization of its gametes occurs in the midgut of female Anopheles mosquito. By inhibiting parasite fertilization in mosquitoes, we can stop malaria parasite transmission from mosquitoes to humans. Using cryo-electron microscopy, we determined the structure of the Pfs230-Pfs48/45 core fertilization complex isolated from the sexual stages of malaria parasites. This structure provided insight on critical domains for complex formation and led to the development of Pfs230 nanobodies and mRNA-LNP that block transmission.
A/Prof. Alastair Stewart
Alastair is a laboratory head at the Victor Chang Cardiac Research Institute in Sydney Australia. Throughout his career he has used a complementary spectrum of structural and biochemical methods to unravel the function of protein complexes important in biomedicine. Alastair embarked on his scientific journey at the University of Cambridge, where he read the Natural Sciences Tripos. He subsequently moved to Sydney to pursue a PhD focused on deciphering the mechanism of ATP synthase. Following his doctoral studies Alastair undertook a brief postdoctoral fellowship at the University of Sydney, delving into the regulation of chromatin by long non-coding RNAs, before setting up his independent research group at the Victor Chang Cardiac Research Institute in 2016. Alastair has been the recipient of three consecutive fellowships from the National Health and Medical Research Council, most recently being awarded the Commonwealth Health Minister’s Award for Excellence in Health and Medical Research for the highest ranked Emerging Leader Investigator Grant in 2023.
Snapshots of Membrane Transport: Sodium-Dependent and Proton-Powered Proteins.
Biological membranes serve as barriers to the external environment, allowing for selective transport and enabling energy conversion through ion gradients. This presentation explores two ion-conducting mechanisms found in membrane proteins: (i) the sodium-dependent human carnitine transporter, OCTN2 and (ii) the proton-powered bacterial ATP synthase.
(i) OCTN2 facilitates carnitine uptake across plasma membranes and is essential for fatty acid metabolism. Mutations in OCTN2 cause systemic primary carnitine deficiency (SPCD), a potentially fatal disorder. Using cryo-EM, we reveal OCTN2 maps under three conditions, identifying a sodium-binding site within an aqueous cavity distinct from the carnitine-binding pocket, as well as molecular transitions during transport. These insights, supported by electrophysiology, define the transport mechanism and provide a framework for understanding SPCD-associated variants.
(ii) ATP synthase couples proton flow to ATP production via rotary catalysis. Here we present multiple cryo-EM maps of the enzyme from Pseudomonas aeruginosa, revealing proton sub-stepping and ordered waters that support a Grotthuss mechanism for proton transfer. These molecular views also reveal separate protonation and deprotonation steps, and deepen our understanding of proton driven ATP synthesis.
Together, these findings offer molecular insights into ion transport and energy conversion, with implications for metabolic disease and antimicrobial drug development.
Program


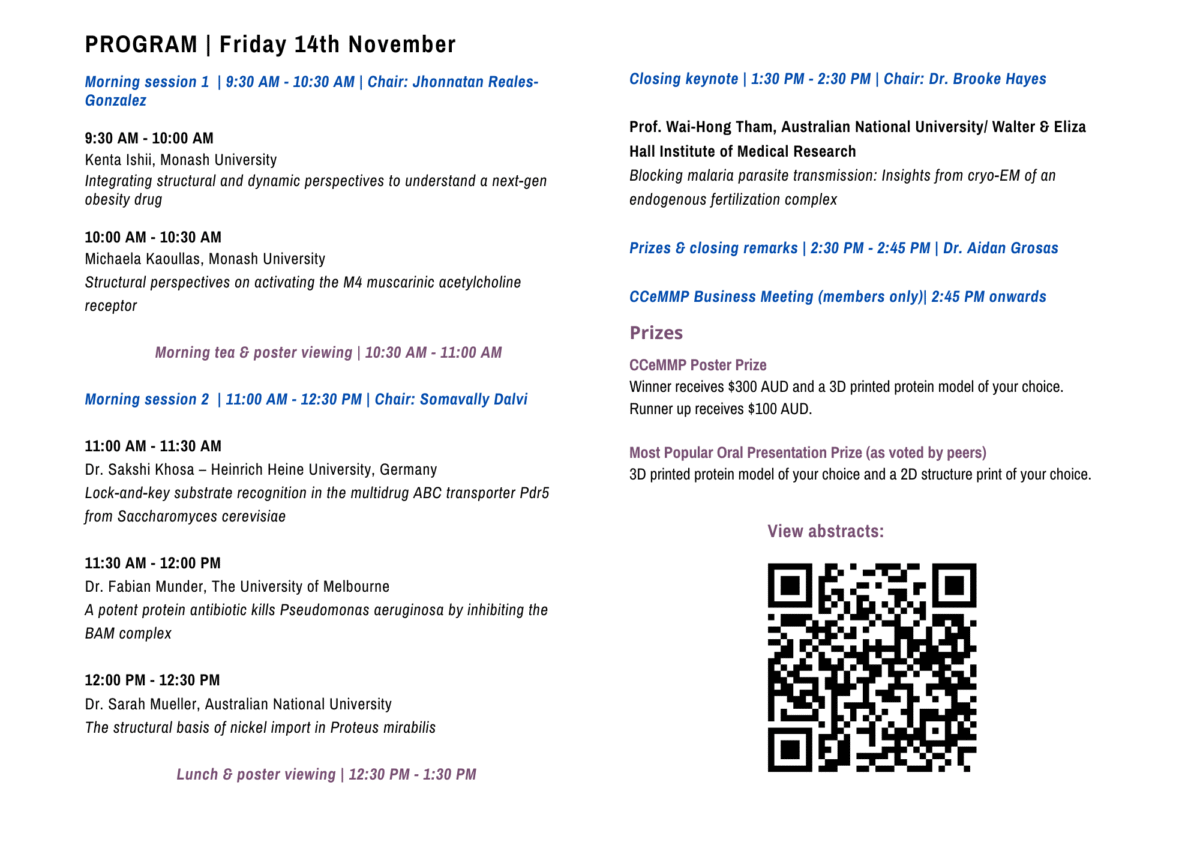
The PDF version of the program will be available here.
Oral Presentation Abstracts
Anna Beyger, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville
Daniel Fox, Bio21, The University of Melbourne, Parkville
AI-designed protein inhibitors can block hemoglobin binding and inhibit growth of pathogenic E. coli
Dr. Rhys Grinter, Bio21, The University of Melbourne, Parkville
Quinone-transporting filaments extend the respiratory chain of Gram-positive bacteria
Dr. Joshua Hardy, The Walter and Eliza Hall Institute of Medical Research, Parkville
ProteinDJ: a high-performance and modular protein design pipeline
Kenta Ishii, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville
Integrating structural and dynamic perspectives to understand a next-gen obesity drug
Javaid Jabbar, Bio21, The University of Melbourne, Parkville
Lysine Acetylation: A switch for OPA1-mediated membrane remodeling
Michaela Kaoullas, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville
Structural perspectives on activating the M4 muscarinic acetylcholine receptor
Dr. Sakshi Khosa, Heinrich Heine University, Duesseldorf, Germany
Dr. Carus Lau, Victor Chang Cardiac Research Institute, Darlinghurst
Probing early inactivation events in hERG using the scorpion toxin CnErg1
Dr. Sarah Mueller, Australian National University, Canberra
The structural basis of nickel import in Proteus mirabilis
Dr. Fabian Munder, Bio21, The University of Melbourne, Parkville
A potent protein antibiotic kills Pseudomonas aeruginosa by inhibiting the BAM complex
Dr. Luca Troman, Bio21, The University of Melbourne, Parkville
The Pam Pilus – a novel pilus system within candidate phyla radiation bacteria
Most Popular Oral Presentation Prize (voted by peers)
Mahmuda Yeasmin, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville
Structural basis of positive allosteric modulator selectivity at the muscarinic acetylcholine receptors
Poster Presentation Abstracts
Poster #1
Dr. Nadezhda Aleksandrova, Bio21, The University of Melbourne, Parkville
Mechanistic insights into the activation and pore formation of insecticidal proteins from ferns
Poster #2
Dr. Rex Anane, Biomedicine Discovery Institute, Monash University, Clayton
Poster #3
Minakshi Baruah, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville
Frozen in action: Capturing vasopressin receptors in their inactive state
Poster #4
Vignesh Kamath Beladi, Bio21, The University of Melbourne, Parkville
Poster #5
Jiahao Chen, Victor Chang Cardiac Research Institute, Darlinghurst
Structural basis of erythromycin binding to hERG K+ channels
Poster #6
Yunzhi Chen, Bio21, The University of Melbourne, Parkville
A cryo-EM structure to unravel the unexpected promiscuity between IL-6 and IL-11Rα
Winner – Poster Prize Poster #7
Somavally Dalvi, Bio21, The University of Melbourne, Parkville
Poster #8
Thomas Ficker, University of Wollongong, Wollongong
Towards the molecular mechanisms of ligand activation in Kv7.5 potassium ion channels
Poster #9
Marialena Georgopoulou, Bio21, The University of Melbourne, Parkville
Runner Up – Poster Prize Poster #10
Dr. Matthew Johnson, Bio21, The University of Melbourne, Parkville
Cryo-Lamella preparation to visualise host pathogen interactions by CryoET
Poster #11
Dr. Bronte Johnstone, Bio21, The University of Melbourne, Parkville
Visualising viral membrane fusion by SARS-CoV-2 using cryo-ET
Poster #12
Riya Joseph, Bio21, The University of Melbourne, Parkville
A structural perspective on pore formation and regulation of Bacteroides fragilis toxins
Poster #13
Mariakatarina Lambourne, University of Wollongong, Wollongong
A twist in the tail: The structural basis of Kv7.2/Kv7.3 channel assembly through helix D
Poster #14
Dr. Bryan Lim, Bio21, The University of Melbourne, Parkville
Unravelling the structural mechanism of the pneumococcal manganese transporter PsaBCA
withdrawn Poster #15
James Lingford, Biomedicine Discovery Institute, Monash University, Clayton
Mapping the unseen structural diversity of hydrogenases in silico
Poster #16
Mayada Mazhar, Bio21, The University of Melbourne, Parkville
Structural and Functional insights on TMEM120A membrane protein
Poster #17
Qinghao Ou, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville
Structural insights into isoquinoline small molecule ligand-induced agonism and modulation on GLP-1R
withdrawn Poster #18
Emily Park, Walter and Eliza Hall Institute of Medical Research, Parkville
Structural basis for non-catalytic signalling mechanisms of Eph receptor pseudokinase EphA10
Poster #19
Dr. Sarah Piper, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville
Structures and dynamics of PAC1 receptor visualised in 3D animations: staying “in the loop”
Poster #20
Inamur Rahman, Bio21, The University of Melbourne, Parkville
Destroying the human immunodeficiency virus before infection
Poster #21
Bhavika Rana, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville
Poster #22
Milad Reyhani, Bio21, The University of Melbourne, Parkville
Structural analysis of new archaeal viruses
Poster #23
David Safadi, University of Wollongong, Wollongong
Structure and function of the GABAB receptor upon the binding and activation by analgesic peptides
Poster #24
Dr. Sepideh Valimehr, Bio21, The University of Melbourne, Parkville
Optimized preparation of graphene-supported grids for high-resolution cryo-EM
Sponsors
This event is generously sponsored by Solve Scientific and Bio21


Organising Committee
2025 Symposium Committee Members
Dr. Ashleigh Kropp, Bio21, The University of Melbourne
Dr. Brooke Hayes, Monash University
Dr. Aidan Grosas, University of Wollongong
Dr. Naveen Vankadari Park, Bio21, The University of Melbourne
Somavally Dalvi, Bio21, The University of Melbourne
Jhonnatan Reales Gonzalez, University of Wollongong
Thomas Ficker, University of Wollongong
Supported by Dr. Tracie Pierce, Monash University
Abstract Submission Guidelines
The abstract submissions for the ARC CCeMMP Research Symposium 2025 are now closed.
Registrations for the Symposium close 5:00 PM (AEDT) October 31st, 2025.
Prizes
- CCeMMP Poster Prize – $300 AUD and a 3D printed protein model of your choice
- CCeMMP Poster Prize Runners-Up – $100 AUD
- Most Popular Oral Presentation Prize (voted by peers) – 3D printed protein model of your choice and a 2D structure print of your choice
Abstract guidelines
Please adhere to the following guidelines when submitting an abstract:
General abstract guidelines
- The submitted abstract should be relevant to the field of research i.e. membrane proteins, cryo-EM/cryo-ET work or cryo-EM/cryo-ET of membrane proteins.
- Abstracts will be maximum 300 words and maximum one A4 page in length, including any figures, photos or graphs.
- Page margins should be a minimum of 2.0 cm.
- All text should use single line spacing and full justification.
- A minimum font size of 12 point in Times New Roman.
- Abstract title should be in bold font.
- Authors should be listed and italicised.
- Address line should be included below the authors names with a space in between author names and address.
- All abstracts must be submitted and presented in English.
- Authors should indicate whether they would like to be considered for an oral presentation in the submission form (select “Poster or Oral”). The selection panel reserves the right to decide which abstracts will be presented in oral form and the length of the oral presentation.
- Upon abstract submission, you will receive an email notifying you of your successful submission.
Submit your abstract here.
Poster presentation guidelines
- Poster presentation abstract submissions have closed.
- All authors submitting abstracts will be able to present a poster.
- Posters must be presented in portrait orientation and must not exceed A0 (841 cm x 1189 cm) size. Equipment to mount posters will be provided on arrival.
- Please indicate in the Abstract Submission Form if you wish to be considered for the CCeMMP Poster Prize.
Oral presentation guidelines
- Oral presentation abstract submissions have closed.
- Please indicate in the Abstract Submission Form if you wish to be considered for an oral presentation (select “Poster or Oral”).
- Abstracts will be reviewed by a selection panel and authors selected for an oral presentation will be notified no later than Friday 17th of October 2025. The selection panel reserves the right to decide which abstracts will be presented in oral form and the length of the oral presentation.
- Oral presentations will be either 10 or 20 min in length followed by question time; you will be informed following abstract selection the length of your presentation.
- Important: Abstracts selected for an oral presentation do not need to present a poster. If your abstract is not selected for an oral presentation, you are required to present a poster; please only submit an abstract if you are also prepared to present a poster.
